
25th European Congress of Psychiatry / European Psychiatry 41S (2017) S238–S302
S283
EW0519
Investigation of salivary cortisol
response to awakening in
underweight and weight-restored
patients with anorexia nervosa
F. Pellegrino
1 ,∗
, A.M. Monteleone
1, M. Nigro
1, V. Ruzzi
1,
M. Cimino
1, U. Volpe
1, P. Monteleone
21
Second University of Naples, Department of Psychiatry, Naples, Italy
2
University of Salerno, Department of Medicine and Surgery, Salerno,
Italy
∗
Corresponding author.
Introduction
Anorexia nervosa (AN) is characterized by dysreg-
ulated eating that leads to chronic malnutrition, which may be
responsible for several physical complications, including endocrine
alterations, such as hyperactivity of the hypothalamus-pituitary-
adrenal (HPA) axis.
Objectives
Several studies have shown a dysregulation of the
cortisol awakening response (CAR) in symptomatic AN patients.
However, it has not been established if the deranged CAR of under-
weight AN patients is a primary phenomenon or an alteration
secondary to malnutrition.
Aims
The aim of this study was to explore the salivary CAR in
both underweight and weight-restored patients with AN.
Methods
We recruited 59 women: 18 undernourished AN
patients, 15 weight-restored AN women and 26 normal-weight
healthy controls. Saliva samples were collected in the morning,
immediately after awakening and after 15, 30 and 60minutes, in
order to measure saliva levels of cortisol. Participants filled in the
state-trait anxiety inventory (STAI) to test their anxiety levels in
the morning of the test.
Results
Compared to healthy controls, underweight AN patients
showed an enhanced CAR whereas the weight recovered patients
had a normal CAR. These results were not correlated with levels of
anxiety.
Conclusions
For the first time, our results demonstrate that the
deranged CAR found in acute AN patients is not present in weight-
restored ones, suggesting that altered activity of the HPA axis of
symptomatic AN patients is a state-dependent phenomenon.
Disclosure of interest
The authors have not supplied their decla-
ration of competing interest.
http://dx.doi.org/10.1016/j.eurpsy.2017.02.133EW0520
Tracking insomnia seasonal variations
through consumption of hypnotics
I.P. Gradiˇski
1 ,∗
, P. Bili´c
2, T. Sabo
2, M. Vilibi´c
21
University Psychiatric Hospital Vrapˇce, Zavod za dualne
poreme´caje, Zagreb, Croatia
2
University Psychiatric Hospital Vrapˇce, Zavod za biologijsku
psihijatriju i psihogerijatriju, Zagreb, Croatia
∗
Corresponding author.
Introduction
Light-stimulated release of melanopsin suppresses
the nocturnal production of melatonin and is sending signals
to multiple brain areas, including hypothalamic suprachiasmatic
nuclei and thus controlling the release of the pineal hormone mela-
tonin and therefore control the circadian rhythm. Consumption
of sedatives and hypnotics was used as an indirect measure of
seasonal variations in sleep disturbances among inpatients at Uni-
versity Psychiatric Hospital Vrapˇce.
Methods
Retrograde record analysis was performed from1st Jan-
uary to 31st December 2012 on commonly used hypnotics and
sedatives: zolpidem, nitrazepam, flurazepam, and midazolam.
Results
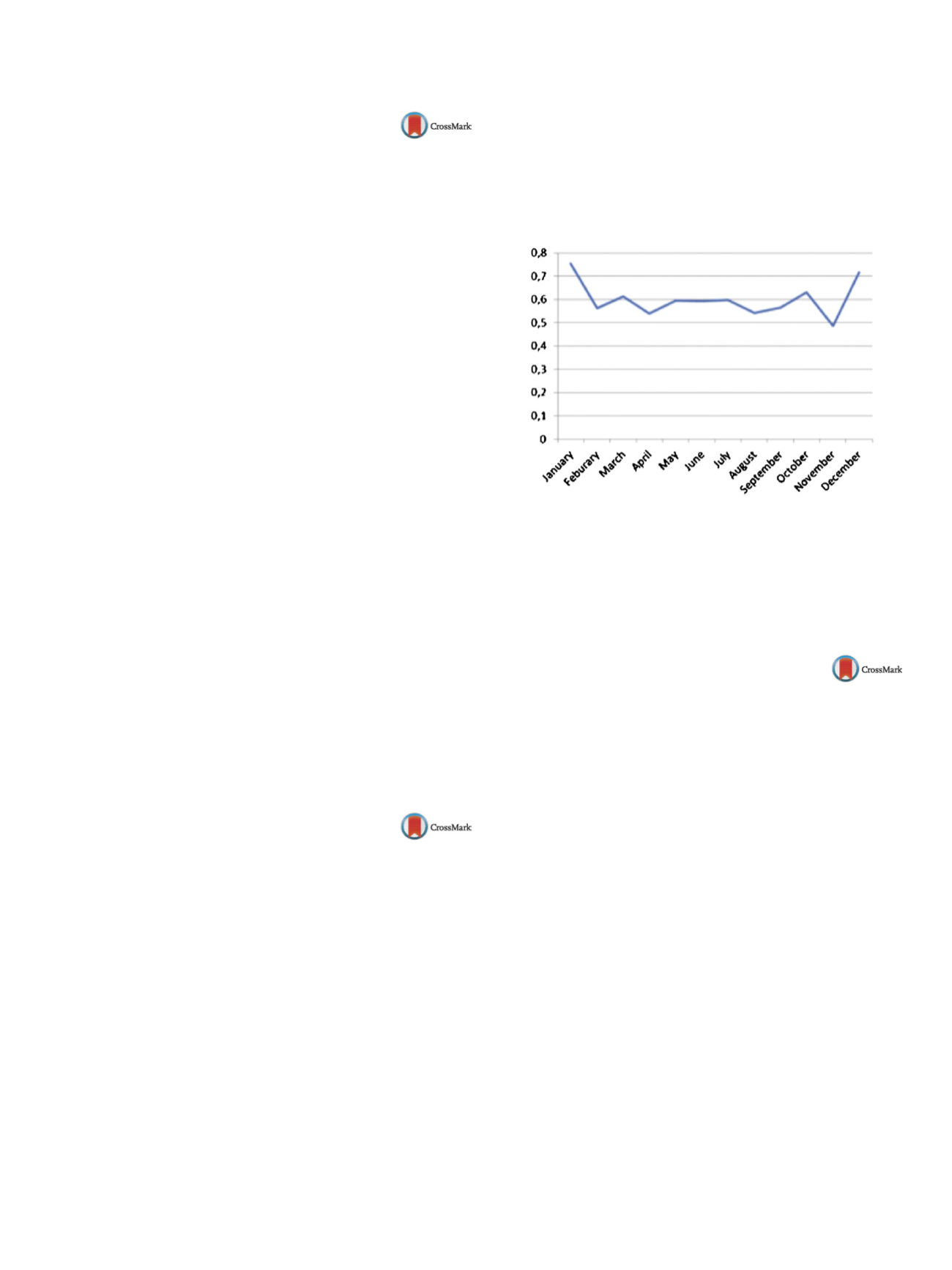
The lowest consumption of hypnotics was recorded in
the months of November, August and September while the highest
consumption was recorded in January, December andMarch which
can be seen in
Fig. 1 . Although therewere differences in themonthly
prescription of hypnotics, when it comes to seasonal patterns, there
are no statistically significant differences.
Conclusions
There is no significant difference between the con-
sumption of hypnotics in the observed seasons although the
consumption of hypnotics is higher in the months with shorter
daylight. This study attempted to correlate exposure to light and
insomnia through the prescription of hypnotics and it is possible
there are other important variables not included in this study.
Fig. 1
Disclosure of interest
The authors have not supplied their decla-
ration of competing interest.
http://dx.doi.org/10.1016/j.eurpsy.2017.02.134EW0521
Antidepressants-induced sexual
troubles
H. Ben Ammar , G. Hamdi
∗
, H. Zalila , Z. El Hechmi
Razi Hospital, F, Mannouba, Tunisia
∗
Corresponding author.
Introduction
For a long time, antidepressants sexual side effects
have been neglected. Currently, no reliable scientific data is avail-
able regarding the nature and frequency of sexual dysfunction
induced by antidepressants. The aim of our study was to evaluate
the prevalence and type of sexual dysfunction induced by antide-
pressants, and to identify factors associated with the occurrence of
these disorders.
Methodology
A descriptive and analytical cross-sectional study
extending over a period of two week. For the purpose of
this research, a socio-demographic card, the Arizona Sexual
Experiences Scale (ASEX) and the Psychotropic-Related Sexual Dys-
function Questionnaire (SALSEX) were used.
Results
Fifty-five patients were recruited. The diagnosis of major
depressive episodes was dominant (49.1%). Moreover, fluoxe-
tine and tricyclic were in top of the list of antidepressants with
respective proportions of 41.8% and 38.2% and respective dose
20.86mg/24 h and 72.38mg/24 h. The score using the ASEX scale
was 14.63
±
5.23. Using the SALSEX scale, 47.3% of patients claimed
to have had sexual disorders secondary to antidepressants with a
moderate score of 9.19
±
2.56. Furthermore, sexual disorders were
more common in the elderly aged of 45 (66.66%) as well as in
patients started on paroxetine (66.66%) and on sertraline (66.66%)
(
P
≤
0.05).
Conclusion
The sexual side effects of antidepressants have a
major impact on the quality of life and adherence to treatment.
They also represent an important risk factor for relapse and recur-
rence in major depression, in this context, the prescription of an
antidepressant.


















