
25th European Congress of Psychiatry / European Psychiatry 41S (2017) S772–S846
S807
EV1232
F17464 a new antipsychotic with
preferential D3 antagonist, 5-HT1A
partial agonist properties.
Neurochemical studies
C. Cosi
∗
, V. N’Guyen , N. Consul-Denjean , A. Auclair , P. Heusler ,
J.C. Martel , L. Leriche , P. Sokoloff , S. Gatti-McArthur
Institut de recherche Pierr-Fabre, centre d’évaluation préclinique,
unitité innovation SNC, Castres, France
∗
Corresponding author.
F17464 is a new dopamine receptor antagonist that recently
demonstrated antipsychotic activity in a proof of concept study
in schizophrenic patients under acute exacerbation. The com-
pound has a unique profile with high affinity for hD
3
receptors
(Ki = 0.17 nM) and lower affinity for hD
2
L (Ki = 12.1 nM) and hD
2
S
(Ki = 6.5 nM). F17464 exhibits also high affinity for h5-HT
1A
recep-
tors (Ki = 0.16 nM). F17464 is a hD
3
antagonist (pK
B
= 9.13), hD
2
S
very week partial agonist (pK
B
= 7.87, emax 8% of DA stimulated
in ERK assay) and a 5-HT
1A
partial agonist (pEC50 = 7.99). F17464
exhibits consistent affinities for rat striatal D
2
(Ki = 4.8 nM) and for
rat hippocampal 5-HT
1A
receptors (Ki = 1.14 nM). Neurochemical
studies show that F17464 ip (1 h post-dose) produces a signifi-
cant dose–dependent increase in the levels of DOPAC and HVA
in the frontal cortex, caudate-putamen and limbic forebrain and
an increase in 3-MT levels in the latter two regions with no
changes in total DA content. The effect is significant at the doses
of 0.63–2.5mg/kg ip (PK/PD data will be provided). This pattern
of DA metabolite changes is similar to that described for several
antipsychotic drugs in rodents and it is indicative of a cortical
effect of F17464. F17464 has a very low cataleptogenic activ-
ity in rats and mice and does not induce serotoninergic signs
typical of 5-HT
1A
. F17464 is therefore a novel a D
3
preferential
antipsychotic with a unique mechanism of action and receptor
affinity profile and a consistent effect in neurochemistry studies in
rodents.
Disclosure of interest
The authors have not supplied their decla-
ration of competing interest.
http://dx.doi.org/10.1016/j.eurpsy.2017.01.1562EV1233
A novel methodology to evaluate the
molecular validity of preclinical
psychosis models compared to
schizophrenia brain pathology
D. Cox
∗
, M. Gottschalk , H. Wesseling , A. Ernst , J. Cooper ,
S. Bahn
Cambridge Centre for Neuropsychiatric Research, Institute of
Biotechnology, Cambridge, United Kingdom
∗
Corresponding author.
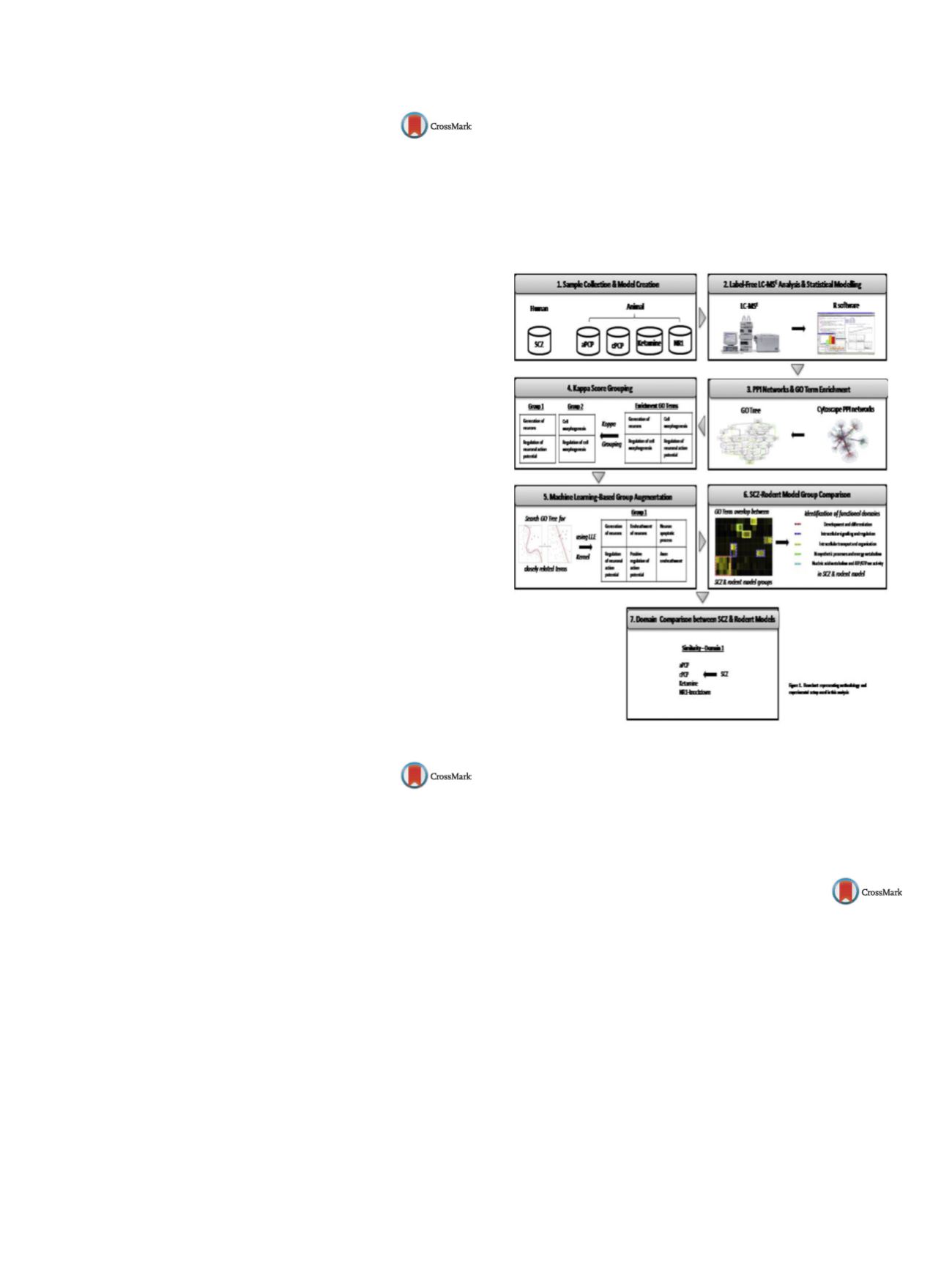
Rodent models of schizophrenia (SCZ) are indispensable when
screening for novel treatments, but quantifying their translational
relevance with the underlying human pathophysiology has proved
difficult. A novel systems methodology (shown in
Figure 1 )was
developed integrating and comparing proteomic data of ante-
rior prefrontal cortex tissue from SCZ post-mortem brains and
matched controls with data obtained from four established glu-
tamatergic rodent models, with the aim of evaluating which of
these models represent SCZ most closely. Liquid chromatography
coupled tandem mass spectrometry (LC-MS
E
) proteomic profil-
ing was applied comparing healthy and “disease state” in human
post-mortem samples and rodent brain tissue samples. Protein-
protein interaction networks were constructed from significant
abundance changes and enrichment analyses enabled the identifi-
cation of pathophysiological characteristics of the disorder, which
were represented across all four rodent models. Subsequently,
these functional domains were used for cross-species comparisons.
Five functional domains such as “development and differentia-
tion” represented across all four rodent models, were identified. It
was quantified that the chronic phencyclidine (cPCP) model repre-
sented SCZ brain changes most closely for four of these functional
domains, by using machine-learning techniques. This is the first
study aiming to quantifywhich rodentmodel recapitulates the neu-
ropathological features of SCZ most closely. The methodology and
findings presented here support recent efforts to overcome trans-
lational hurdles of preclinical psychiatric research by associating
behavioural endophenotypes with distinct biological processes.
Fig. 1
Disclosure of interest
The authors have not supplied their decla-
ration of competing interest.
http://dx.doi.org/10.1016/j.eurpsy.2017.01.1563EV1234
The geometrical analysis of
handwriting as a new tool to evaluate
motor symptoms in psychosis
Y. Crespo Cobo
1 ,∗
, A. Iba˜nez Molina
2, S. Iglesias Parro
2,
M.F. Soriano Pe˜na
3 , J.I.Aznarte
31
FIBAO, Psychology, Jaén, Spain
2
University of Jaén, Psychology, Jaén, Spain
3
Hospital San Agustín, Mental Health Unit, Linares, Spain
∗
Corresponding author.
Introduction
There is growing evidence about the importance
of motor symptoms in psychosis. Motor abnormalities have been
observed in naive-drugs, first-episode patients. Clinical assessment
of motor abnormalities normally relies upon subjective observer-
based ratings. Kinematic analysis of handwriting has proved to be
an objective measure of motor symptoms, but it has not been used
in clinical settings.
Objectives
In the present work, the geometrical analysis of hand-
writing patterns is proposed as a new tool to evaluate motor
symptoms in psychosis.


















